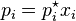Limiting reagent(L.R.) is a reagent which is consumed earlier in a chemical reaction.
Limiting reagent determines the amount of product formed in a given chemical reaction, the maximum amount of product formed is the amount formed by the L.R.
Identification of L.R.
4A + 3B -----------> 5C
5 4
Divide the given number of moles by respective stoichiometric coefficients
5/4 = 1.25 4/3 = 1.33
Reactant having lesser value of the two will be the L.R. Thus in the above reaction A is the L.R. and B is Excess Reagent (E.R.).
Moles of excess reagent left = Initial moles of E.R. - Coefficient of E.R./Coefficient of L.R.* Initial
moles of L.R.
i.e. Amount of B left = 4-3/4*5 = 4 - 3.75 = 0.25 mole
Moles of product formed = Initial moles of product - Coefficient of product/Coefficient of L.R.* Initial moles of L.R.
i.e. Amount of C left = 5/4 * 5 = 25/4 = 6.25 mole
Remarks:- Use this special trick for faster calculations . You can solve any question of L.R. within 30-40 seconds once you have practiced this trick properly.