RAOULT'S LAW- The partial pressure of any volatile constituent of a solution at constant temperature is equal to the product of vapour pressure of pure constituent and its mole fraction.
i.e.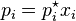
Where Pi = Vapoue pressure of solution, p*= V.P. of pure Constituent, xi = Mole fraction of Constituent
1. Raoult's law for binary solution of two volatile liquids:- If A is solute and B is solvent then

The figure shows the variation in V.P. with varying composition of the mixture.
2.Solid liquid solution containing non- volatile solute -
The V.P. of a solution containing non- volatile solute is directly proportional to the mole fraction of the Solvent.
Ps = p0 XB
The vapour pressure of a pure solvent is always greater than the vapour pressure of solution contaning non- volatile solute.
Raoult's law is applicable only for dilute solution containing non volatile solute only.
Problem for practice-
Q. Benzene and Toluene form an Ideal solution. The vapour pressure of benzene and Toluene are 75mm and 22mm Hg respectively. If the mole fraction of benzene and toluene in vapour state are 0.63 and 0.37 respectively than calculate the vapour pressure of the ideal mixture. (Ans- 39.68mm of Hg)
i.e.
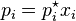
Where Pi = Vapoue pressure of solution, p*= V.P. of pure Constituent, xi = Mole fraction of Constituent
1. Raoult's law for binary solution of two volatile liquids:- If A is solute and B is solvent then

Once the components in the solution have reached equilibrium, the total vapor pressure p of the solution is:
The figure shows the variation in V.P. with varying composition of the mixture.
2.Solid liquid solution containing non- volatile solute -
The V.P. of a solution containing non- volatile solute is directly proportional to the mole fraction of the Solvent.
Ps = p0 XB
The vapour pressure of a pure solvent is always greater than the vapour pressure of solution contaning non- volatile solute.
Raoult's law is applicable only for dilute solution containing non volatile solute only.
Problem for practice-
Q. Benzene and Toluene form an Ideal solution. The vapour pressure of benzene and Toluene are 75mm and 22mm Hg respectively. If the mole fraction of benzene and toluene in vapour state are 0.63 and 0.37 respectively than calculate the vapour pressure of the ideal mixture. (Ans- 39.68mm of Hg)

No comments:
Post a Comment